

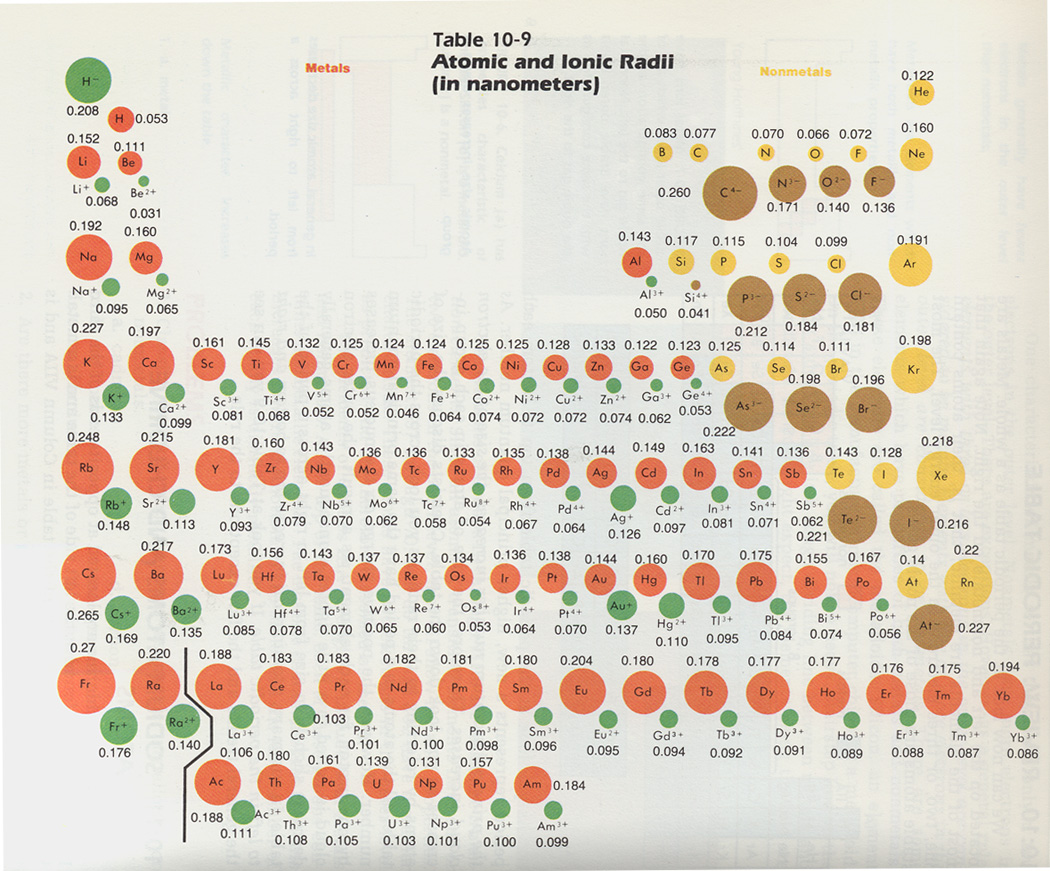
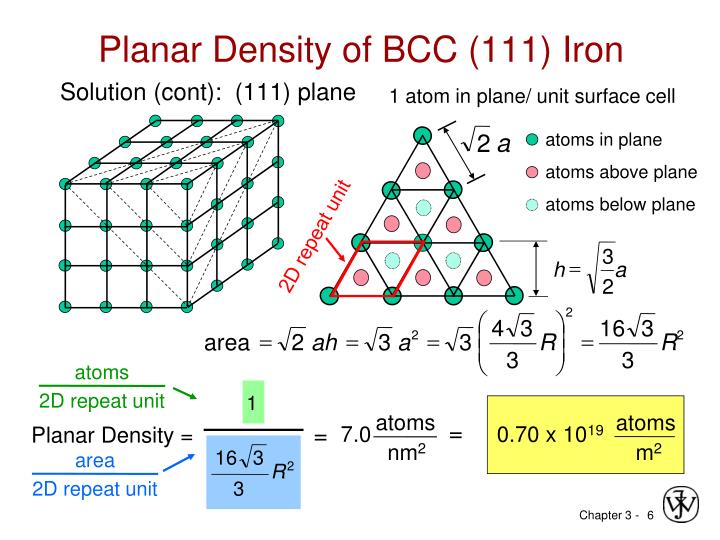
Copper compounds, such as Fehling's solution, are widely used in analytical chemistry tests for sugar. First, consider the atomic-site unit cell for the BCC crystal structure shown below in. Body-Centered Cubic (BCC) Crystal Structure. The atomic radii of selected metals are listed in Tables respectively for the BCC, FCC, and HCP. Shannon, Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides. The radius of the aluminum atom in the aluminum metal is assumed to be half the interatomic distance of 0.143 nm. Iron's alloys - brass and bronze - are very important: all American coins are copper alloys and gun metals also contain copper.Ĭopper has wide use as an agricultural poison and as an algaecide in water purification. The data contained in the database was taken from: R.D. The electrical industry is one of the greatest users of copper. Each carbon atom occupies a position at the center of a trigonal prism of six iron atoms, with the Fe-C distance 2.01 0.01 A. Atomic radius values do not systematically increase or systematically decrease as we move across the row of. From these, copper is obtained by smelting, leaching, and by electrolysis. The most important copper ores are the sulfides, the oxides, and carbonates. Large copper ore deposits are found in the U.S., Chile, Zambia, Zaire, Peru, and Canada. Simply put, the atomic radius is half of the diameter of the atom, which is a result of the number of protons, neutrons, and electrons that compose the atom. Up to date, curated data provided by Mathematica 's ElementData function from Wolfram Research, Inc. SourcesĬopper occasionally occurs natively, and is found in many minerals such as cuprite, malachite, azurite, chalcopyrite, and bornite.
It is malleable, ductile, and a good conductor of heat and electricity (second only to silver in electrical conductivity). PropertiesĬopper is reddish and takes on a bright metallic luster. It is believed that copper has been mined for 5,000 years. −2, +1, +2, +3, +4 (a mildly basic oxide)įrom the Latin word cuprum, from the island of Cyprus. the electron configuration of Fe is Ar3d64s2, whereas that of Fe2+ is Ar3d6. Copper is often used for electrical wiring applications and for household plumbing applications. Some of these properties, such as ionization energy and atomic radius.


 0 kommentar(er)
0 kommentar(er)
